A quantity Q of heat is removed from each cube. B sublimes.

Thermodynamic Terms And Basic Concepts Physical Chemistry Chemical Changes Chemistry
A phase change or transition occurs when a substance undergoes a change in state on a molecular level.

. This is a different temperature for every substance. For example imagine we had water ice at -10 circ C and we wanted to convert. When a pure substance undergoes a chemical change it is no longer that same substance.
In most substances changes in temperature or pressure result in a substance phase change. D vaporizes. The amount of energy necessary to produce sublimation is not too surprisingly called the enthalpy of sublimation.
This preview shows page 6 - 8 out of 10 pages. The substance must be a gas. We review their content and use your feedback to keep the quality high.
Questo 2011 polnt Saved Complete the following statement. D vaporizes. The substance must be cooler than its environment.
A phase change or transition occurs when a substance undergoes a change in state on a molecular level. A freezes. The substance undergoes a change of phase.
Two cubes one silver and one iron have the same mass and temperature. Complete the following statement. Answer 1 of 3.
By Calia Roberts. The substance undergoes a change of phase. It may take extreme temperature pressure or energy but all matter can be changed.
Fusion is a physical process. The substance must be cooler than its environment. A freezes.
A substance changes from a solid to a liquid at the substances melting point. Which one of the following properties causes the. Answer 1 of 2.
In most substances changes in temperature or pressure result in a substance phase change. Complete the following statement. For example water ice melts at 0oC whereas gold melts at 1.
Total Heat Needed to Change Temperature and Phase for a Substance. A phase is a distinctive form of a substance and matter can change among the phases. For eg for the conversion of water into its vapour stateits molecules absorb heat energy and start moving more randomly and in more intense ma.
When a substance undergoes fusion it a Evaporates O b Freezes O c Condenses d Vaporizes e Sublimes. The substance must be a non-perfect solid. B sublimes.
In most substances changes in temperature or pressure result in a substance phase change. A chemical change changes the identity of the substance. When a substance undergoes a change of state it can use energy or give off energy.
For a given substance the sum of its enthalpy of fusion and enthalpy of vaporization is approximately equal to its enthalpy of sublimation. Experts are tested by Chegg as specialists in their subject area. The process of fusion of substances takes place during the conversion of a solid substance into a liquid.
The substance has unusual thermal properties. There is one oddball - The enthalpy of fusion is almost always a positive quantity. When a substance undergoes fusion it vaporizes.
When a substance undergoes a phase changeChanges occur in the intermolecular forces of attraction and hence the intermolecular space. When a substance undergoes fusion it. Heat is added to a substance but its temperature does not rise.
For this process to take place heat is being applied to the solid substance and the particles of the solid loose then move freely thereby reducing the intermolecular forces in the. A phase change or transition occurs when a substance undergoes a change in state on a molecular level. E evaporates.
A substances ability to resist being scratched is the physical property of matter known as. There are several processes of phase changes including fusion solidification vaporization condensation sublimation and physical vapor deposition. C condenses.
The change that occurs when a substance changes from a gas to a solid is referred to as. There is alway energy input to initiate phase change It can be positive as in change from water liquid to gas or negative - gas to liquid. Complete the following statement when a substance.
This is the amount of energy needed to change the total interal energy ie enthalpy of a substance in order to produce the phase transition. When a substance undergoes fusion it melts. There are several processes of phase changes including fusion solidification vaporization condensation sublimation and.
There are several processes of phase changes including fusion solidification vaporization condensation sublimation and physical. C condenses. 100 4 ratings Transcribed image text.
Plateaus in the curve regions of constant temperature are exhibited when the substance undergoes phase transitions. Complete the following statement. When a substance undergoes fusion it.
Which one of the following statements provides the best explanation for this observation.

Complete The Following Statement When A Substance Undergoes Fusion It A Freezes Course Hero

Orbital Representation Of Molecules Molecules Physical Chemistry Electron Configuration

Heat Of Combustion Physical Chemistry Chemistry Chemical Equation
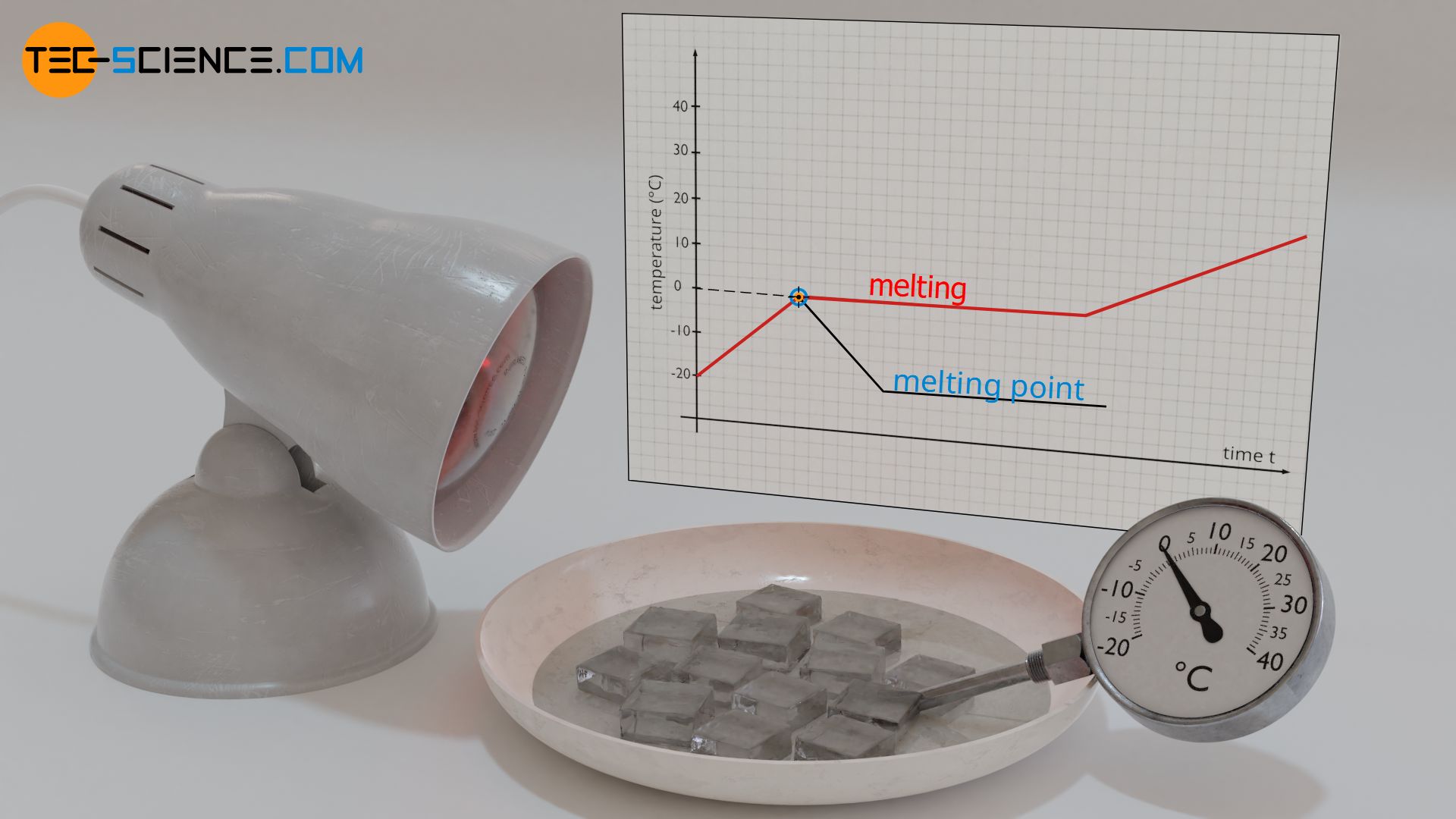
Why Does The Temperature Remain Constant During A Change Of State Phase Transition Tec Science
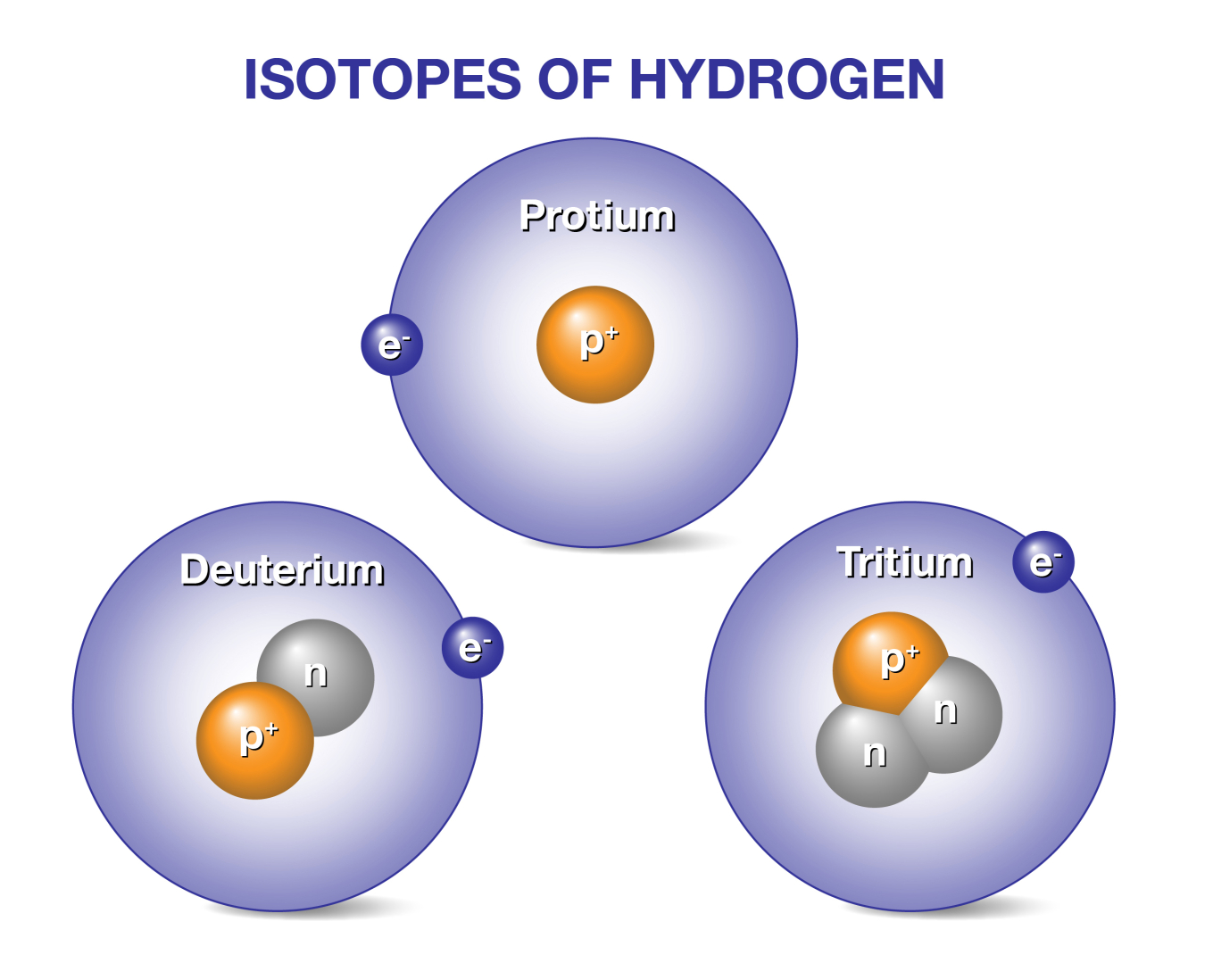
Doe Explains Deuterium Tritium Fusion Reactor Fuel Department Of Energy

Hess S Law Chemical Changes Chemistry Physical Chemistry
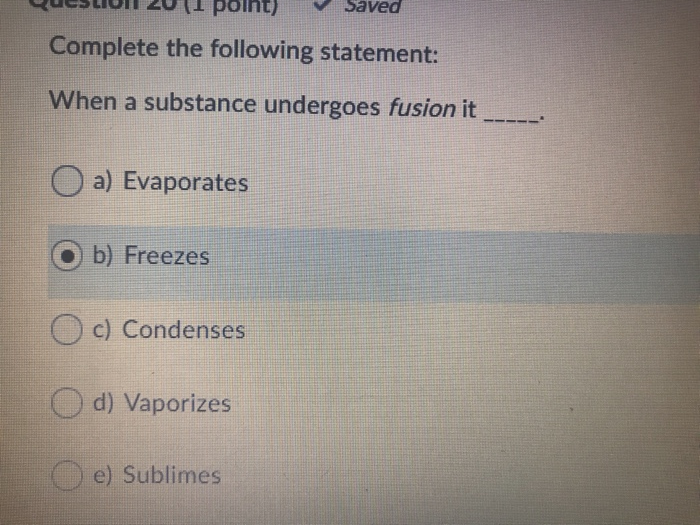
Solved Questo 2011 Polnt Saved Complete The Following Chegg Com

Specific Heat Heat Of Fusion And Vaporization Example Video Khan Academy

Radiation Caution Sign Green Thorium Nuclear Energy Pie Chart

Complete The Following Statement When A Substance Undergoes Fusion It A Freezes Course Hero

Intermolecular Force Easy Science Intermolecular Force Force Definition Chemical Changes

Thermodynamic Processes Chemistry Physical Chemistry Reversible Process

Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09

Chemistry Lab Heat Of Crystallization Classroom Freebies Chemistry Labs Chemistry Classroom Teaching Chemistry

Lab Heat Of Crystallization Classroom Freebies Chemistry Classroom Chemistry Labs Teaching Chemistry





